Submitting Protocols for Review
If your research meets any of the above definitions you must submit protocols for those projects to the IBC for review, regardless of funding. Effective February 1, 2019, all IBC submissions must be made electronically via GW iRIS.
Logging into the IBC Module of GW iRIS
GW faculty/staff/students can log into GW iRIS by visiting https://gwu-iris.imedris.net and entering their NetID and password. All other users should consult the Reference Guide: Logging In for the First Time (PDF).
How to Submit a Protocol
To submit a new protocol, log into GW iRIS and choose the following path:
- Study Assistant > Add a New Study > GW IBC Protocol
- Step-by-step reference guide for submitting a protocol
Submitting Amendments/Terminations/Renewals/Incident Reports for an IBC Protocol
To submit an amendment/termination/annual renewal/incident report, log into GW iRIS and choose the following path:
- Option 1: Study Assistant > Submit a Form > Open the study for which you’d like to submit a form
- Option 2: Study Assistant > My Studies > Open the study for which you’d like to submit a form
- Step-by-step reference guide for submitting amendments/terminations/renewals/incident reports
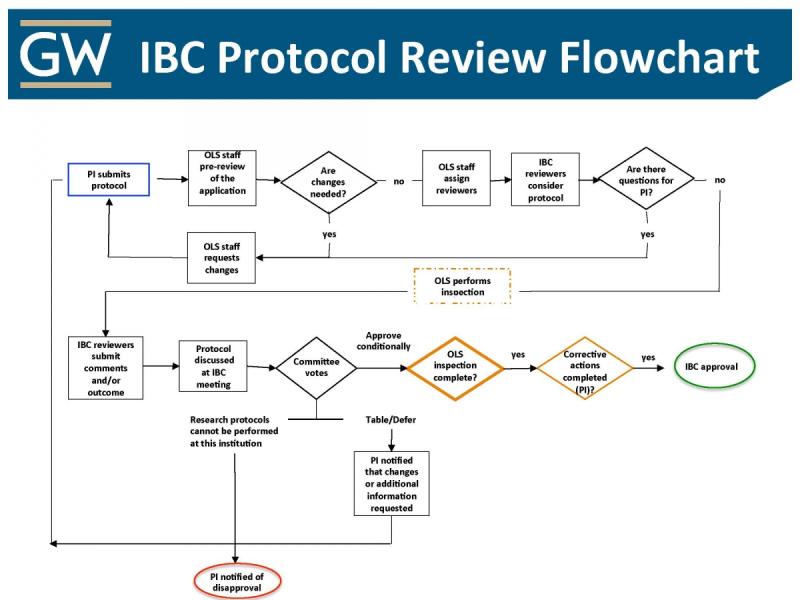
IBC Protocol Review Flowchart
The flowchart below serves as a guide on how the IBC review process works after a PI submits a protocol.
GW iRIS Training Videos
- How to Submit an Application
- Submitting a Modification/Amendment
Templates & SOP Example
The following templates may be required as you prepare your IBC submission. Completed documents can be submitted as attachments within GW iRIS.
- Lentiviral Vector Safety Manual Template (DOC) (if needed)
- Biosafety Level 2 Manual Template (DOC) (if needed)
- Biosafety SOP Worksheet Example
- Biosafety Level 2 with Enhanced Practices (BSL-2+) guidelines (DOC)